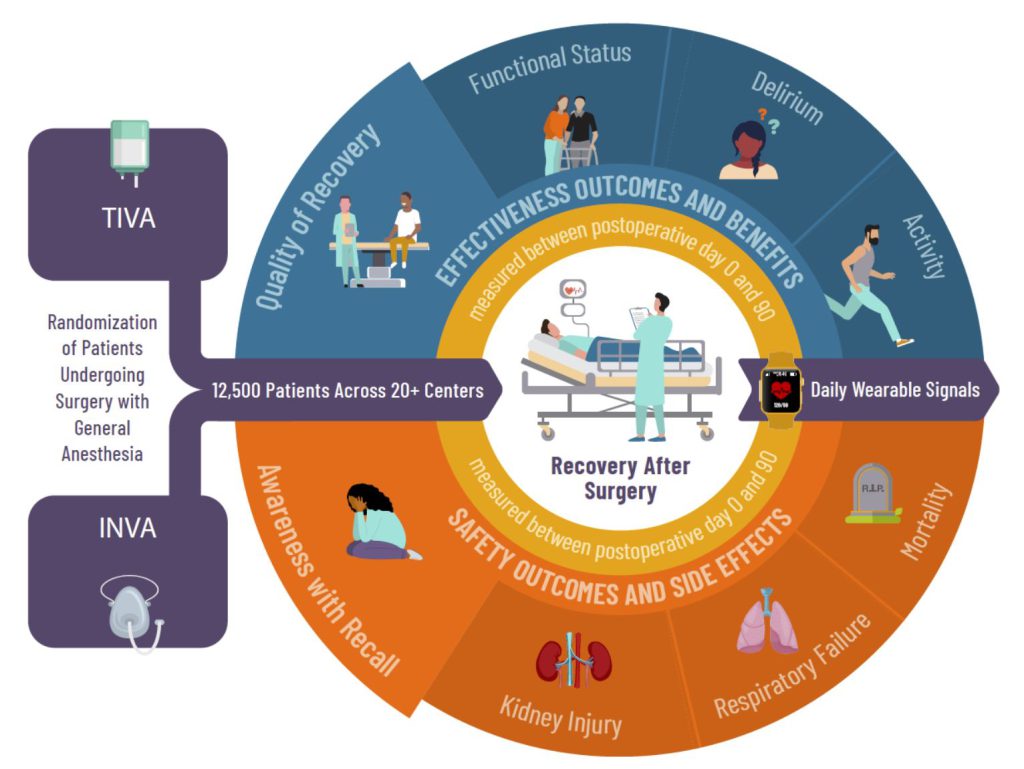
Please review our other website pages to learn more about the structure of the THRIVE trial, view our anticipated timeline, read our monthly newsletters, and view additional clinician educational resources.
More information regarding becoming a THRIVE
enrollment site can be found on the
Information for Interested Sites page.
For general THRIVE inquiries please contact askthrive@umich.edu. If you need to reach out to a specific THRIVE team member, please refer to
the Operational Structure webpage for contact information.